Chapter 6 Elementary Functions
Section 6.4 Exponential and Logarithmic Functions6.4.3 The Natural Exponential Function
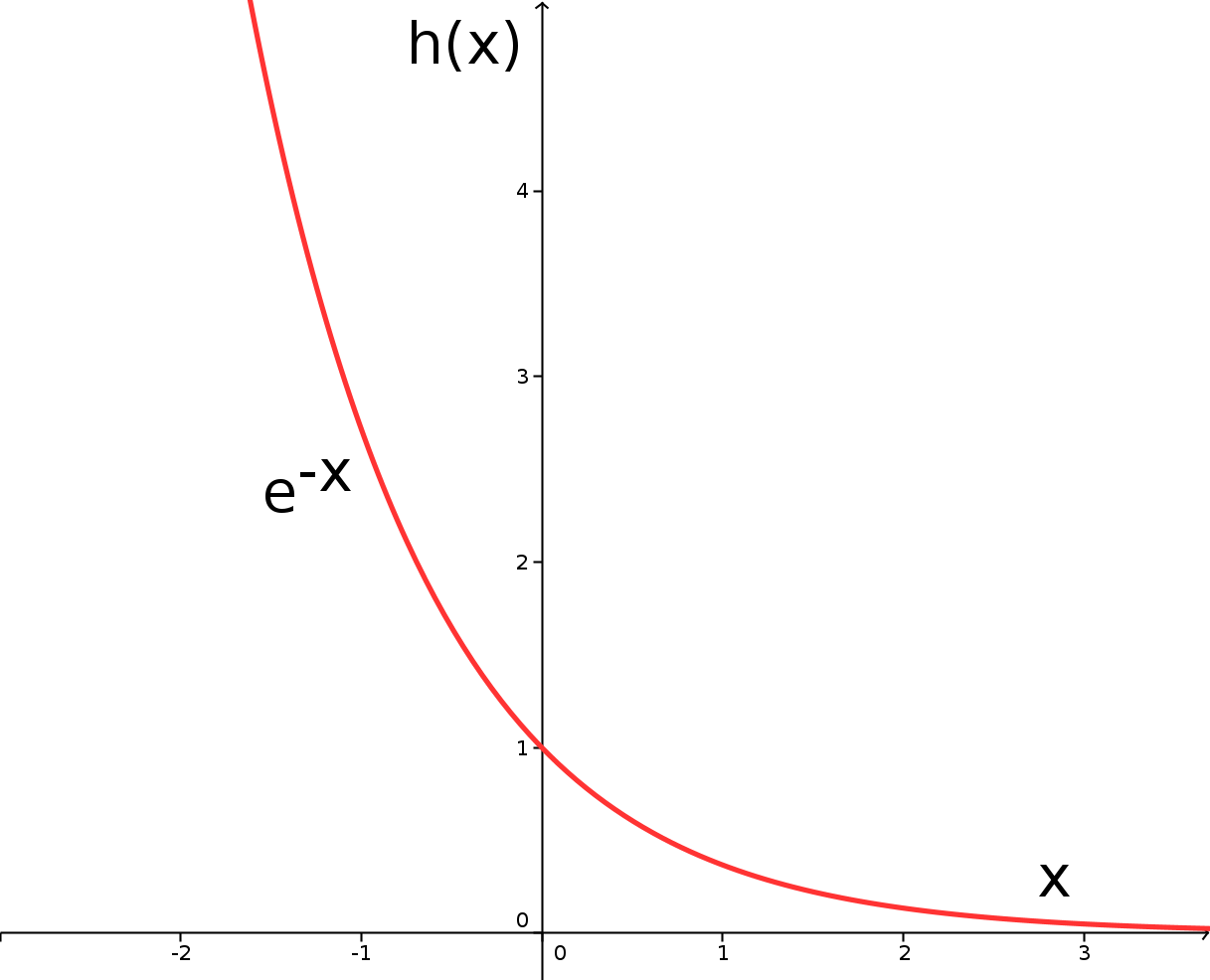
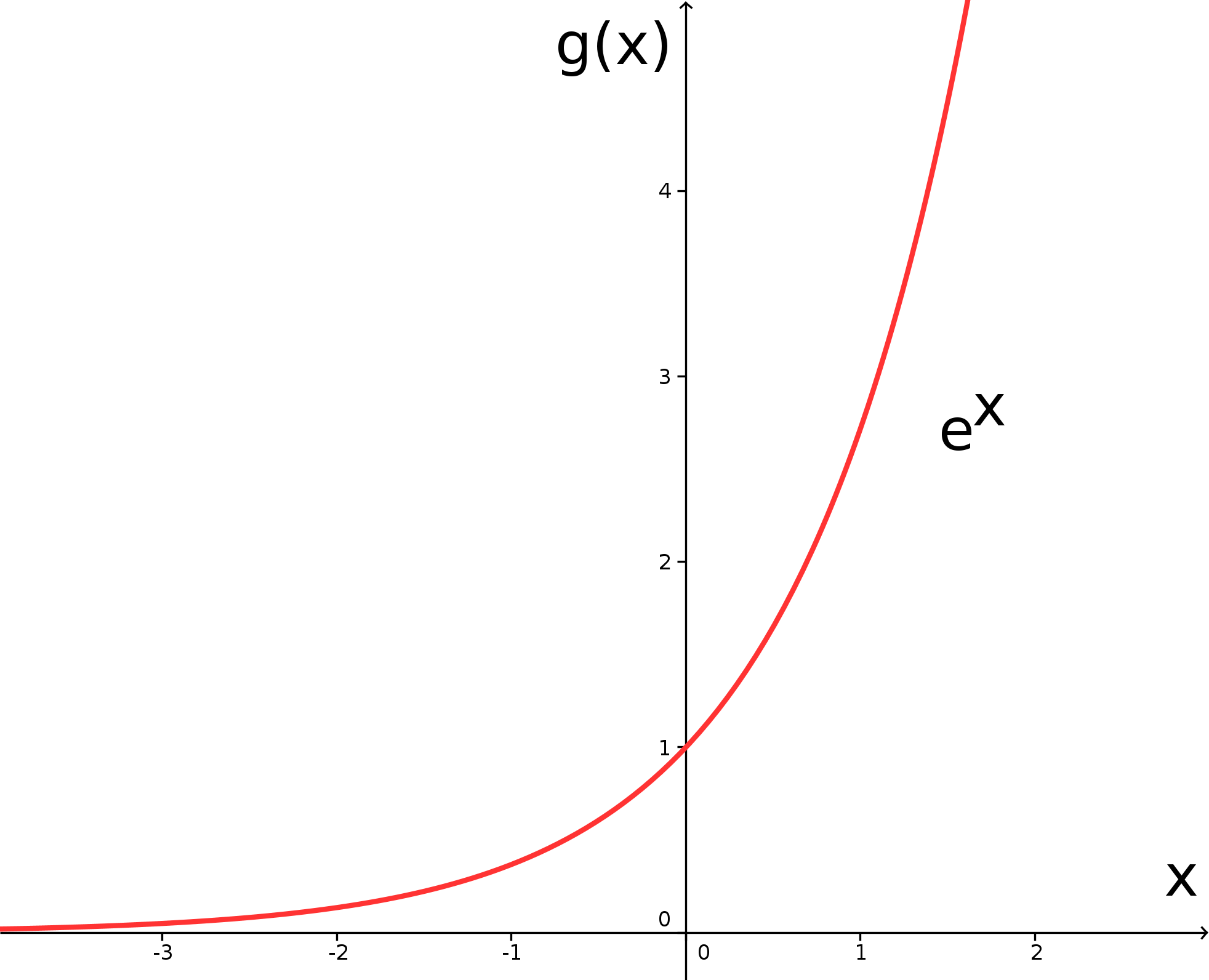
There is a very special exponential function, sometimes also called the exponential function, that we will study now. In fact, all other exponential functions can be reduced to this special exponential function. It has Euler's number as its base. Its value is (approximately) equal toSo, let us consider the graph of the exponential function - for the time being without any additional parameters -
which is, because of its base , also called the function or natural exponential function:
At the beginning of this subsection we claimed that the exponential functions described above can be reduced to the natural exponential function. This is done by means of the identity
that is valid for any real number and any real number . Here, denotes the natural logarithmic function that will be studied in detail in the following Section 6.4.4.
Exercise 6.4.5
Explain why the identity is valid.
In general natural exponential functions, the parameters and occur that were already introduced in Section 6.4.2; thus, its functional description is as follows:
Again, the parameter describes initial values different from , and the factor in the exponent allows for different (positive or negative) growth rates. This shall be finally illustrated by means of an example.
Example 6.4.6
A series of experiments with radioactive iodine atoms () results in the following mean data:
In other words: every days the number of iodine atoms halves due to radioactive decay. For this reason one says in this context that the half-life of equals days.
The radioactive decay follows an exponential law:
Our exponential function is here denoted by ; it describes the number of remaining iodine atoms. Accordingly, denotes the number of iodine atoms at the beginning, i.e. . The independent variable is in this case the time (measured in days). We expect the parameter to be negative since the exponential function describes a decay process, i.e. a process with a negative growth rate. We will determine from the measurement data.
After days only iodine atoms are still present, i.e. . Using the exponential law for the radioactive decay, we obtain:
Now, we can cancel on both sides of the equation and subsequently take the natural logarithm of the equation (see Section 6.4.4):
We transform the left hand side according to the calculation rules for logarithmic functions (see Section 6.4.4): . For the right hand side we note that the natural logarithmic function is the inverse function of the natural exponential function, i.e. ; thus we have:
Inserting the half-life days of results in this case in
Other radioactive substances have different half-lifes, e.g. has a half-life of years, and hence they result in different values of the parameter in the exponential law for the radioactive decay.
| Number of Iodine Atoms | 10 000 | 5 000 | 2 500 | 1 250 | etc. |
| Number of Days Elapsed | 0 | etc. |
The radioactive decay follows an exponential law:
Our exponential function is here denoted by ; it describes the number of remaining iodine atoms. Accordingly, denotes the number of iodine atoms at the beginning, i.e. . The independent variable is in this case the time (measured in days). We expect the parameter to be negative since the exponential function describes a decay process, i.e. a process with a negative growth rate. We will determine from the measurement data.
After days only iodine atoms are still present, i.e. . Using the exponential law for the radioactive decay, we obtain:
Now, we can cancel on both sides of the equation and subsequently take the natural logarithm of the equation (see Section 6.4.4):
We transform the left hand side according to the calculation rules for logarithmic functions (see Section 6.4.4): . For the right hand side we note that the natural logarithmic function is the inverse function of the natural exponential function, i.e. ; thus we have:
Inserting the half-life days of results in this case in
Other radioactive substances have different half-lifes, e.g. has a half-life of years, and hence they result in different values of the parameter in the exponential law for the radioactive decay.
 Onlinebrückenkurs Mathematik
Onlinebrückenkurs Mathematik